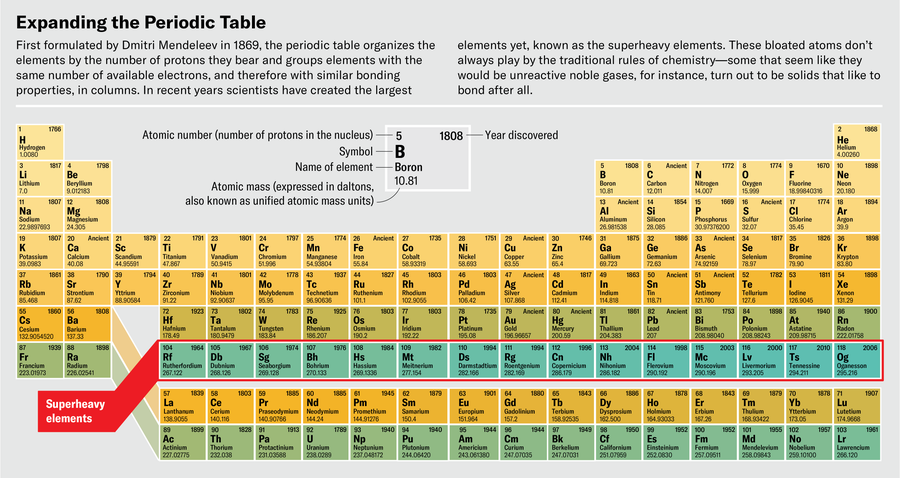
At the far end of the periodic table is a realm where nothing is quite as it should be. The elements here, starting at atomic number 104 (rutherfordium), have never been found in nature. In fact, they’d emphatically prefer not to exist. Their nuclei, bursting with protons and neutrons, tear themselves apart via fission or radioactive decay within instants of their creation.
These are the superheavy elements: after rutherfordium come dubnium, seaborgium, bohrium, and other oddities, all the way up to the heaviest element ever created, oganesson, element 118. Humans have only ever made vanishingly small amounts of these elements. As of 2020, 18 years after the first successful creation of oganesson in a laboratory, scientists had reported making a total of five atoms of it. Even if they could make much more, it would never be the kind of stuff you could hold in your hand—oganesson is so radioactive that it would be less matter, more heat.
Using ultrafast, atom-at-a-time methods, researchers are starting to explore this unmapped region of the periodic table and finding it as fantastical as any medieval cartographer’s imaginings. Here at the uncharted coastline of chemistry, atoms have a host of weird properties, from pumpkin-shaped nuclei to electrons bound so tightly to the nucleus they’re subject to the rules of relativity, not unlike objects orbiting a black hole.
On supporting science journalism
If you're enjoying this article, consider supporting our award-winning journalism by subscribing. By purchasing a subscription you are helping to ensure the future of impactful stories about the discoveries and ideas shaping our world today.
Their properties may reveal more about the primordial elements created in massive astrophysical phenomena such as supernovae and neutron star mergers. But more than that, studying this strange matter may help scientists understand the more typical matter that occurs naturally all around us. As researchers get better at pinning these atoms down and measuring them, they’re pushing the boundaries of the way we organize matter in the first place.
“The periodic table is something fundamental,” says Witold Nazarewicz, a theoretical nuclear physicist and chief scientist at the Facility for Rare Isotope Beams at Michigan State University. “What are the limits of this concept? What are the limits of atomic physics? Where is the end of chemistry?”
Affixed to the wall in a concrete-block corridor known as Cave 1 in Lawrence Berkeley National Laboratory (LBNL), just steps from one of the few instruments in the world that can create superheavy atoms, is a poster-size printout of a table that organizes elements by nuclide, meaning based on the number of protons and neutrons in the nucleus. This graph shows all the known information about the nuclear structure and decay of the elements, as well as of their isotopes—variations on elements with the same number of protons in the nucleus but different numbers of neutrons.
It’s a living document. There’s a typo in the title, and there are tears along the poster’s edges where duct tape holds it to the wall. It’s been marked up with notations in Sharpie, added after the poster was printed in 2006. These notations are the atomic physics version of seafarers penciling in new islands as they sail, but in this case, the islands are isotopes of elements so heavy they can be seen only in particle accelerators like the one here. In a field where it can take a week to make just one atom of what you want, a record of progress is essential.
“Everybody likes the handwritten part,” says Jacklyn Gates, who leads LBNL’s Heavy Element Group. “If we were to print this out from 2023—”
“It’s not as fun,” chimes in Jennifer Pore, a staff scientist in the lab.
“It’s not as fun,” Gates agrees.
Gates is a nuclear chemist with a wry sense of humor and a clear fondness for the equipment that she and her team have developed to synthesize superheavy elements. They create these elements by smashing standard-size atoms together in a 2.2-meter-wide cyclotron—a drum-shaped particle accelerator—in a lab perched on a hillside above the city of Berkeley. Construction on the cyclotron started in 1958, after the fallout from the first nuclear bomb explosions began turning up in the form of new radioactive elements such as fermium (atomic number 100). Much of the original cyclotron persists today; in the control room, silver dials that wouldn’t be out of place in a cold war–era thriller sit beside beige panels from the 1980s and blue banks of buttons from modern updates.
The first of the superheavies, rutherfordium, was synthesized here in 1969. Rutherfordium, named after Ernest Rutherford, who helped to explain the structure of atoms, was also made a few years prior by the Russian Joint Institute for Nuclear Research (JINR) in Dubna, the same group that first created oganesson in 2002 (named after Yuri Oganessian, who led the team that created it). Beginning in the late 1950s, the competition to add new elements got hotter than the ion beams used to make them. Today the vicious disputes over who synthesized what first, mostly between the Berkeley lab and JINR, are remembered as the Transferium Wars.
By the 1980s Germany had joined the fray with its nuclear research institute, Gesellschaft für Schwerionenforschung (GSI), or the Society for Heavy Ion Research. The numbers ticked higher, with the three teams trading off naming rights up to copernicium (element 112, named after Nicolaus Copernicus), discovered in 1996. Controversy continued to dog the superheavies; in 1999 researchers at LBNL announced the discovery of element 116, now known as livermorium after Lawrence Livermore National Laboratory, only to retract that claim after finding that one of their scientists had fabricated evidence. (JINR successfully created livermorium in 2000.) In 2004 Japan’s Institute of Physical and Chemical Research (RIKEN) synthesized element 113, nihonium, after the Japanese word for “Japan.” Although element 118 is the heaviest element ever synthesized, the most recently discovered is actually 117, tennessine, which was announced by JINR in 2010. The scientists behind the discovery named it in tribute to the state of Tennessee, home to several institutions that played a role in the experiments.
“What are the limits of atomic physics? Where is the end of chemistry?”
—Witold Nazarewicz Michigan State University
The race to create ever heavier elements continues to this day, and not just because the researchers who succeed get to name a new element in the periodic table. It’s also because theorists predict that certain combinations of protons and neutrons may land in an “island of stability” where these elements will stop decaying immediately. “Some theories predict a year half-life, or 100 or 1,000 days,” says Hiromitsu Haba, a physicist and director of the Nuclear Chemistry Group at RIKEN, which is currently on the hunt for element 119.
A half-life—the time it takes for about half of a substance’s atoms to decay—that long would be enough for serious experimentation or even use in new technologies. For now, though, research into superheavies is focused on their fundamental properties and what they can reveal about nuclear dynamics, not what they can do as materials themselves. That doesn’t mean they won’t eventually become useful, however.
“Everything we’re doing right now ... it doesn’t have practical applications,” Gates says. “But if you look at your cell phone and all the technology that went into that—that technology started back in the Bronze Age. People didn’t know it would result in these devices that we’re all glued to and utterly dependent on. So can superheavy elements be useful? Maybe not in my generation but maybe a generation or two down the road, when we have better technology and can make these things a little bit easier.”
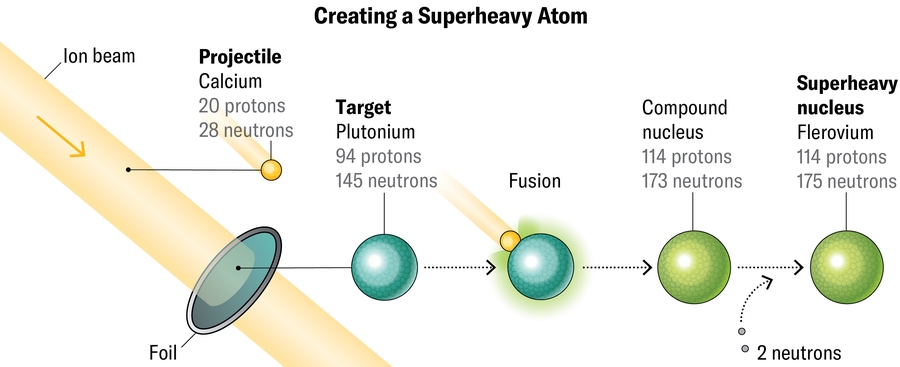
Making these elements is far from easy. Researchers do it by shooting a beam of heavy ions (in this case, large atomic nuclei without their electrons) at a target material in the hopes of overcoming the electrostatic repulsion between two positively charged nuclei and forcing them to fuse. At LBNL, the source of the ion beam is a device called VENUS (for “versatile electron cyclotron resonance ion source for nuclear science”), which sits at the top of the cyclotron behind fencing festooned with radiation warnings. Within VENUS, a combination of microwaves and strong magnetic fields strips electrons off a chosen element (often calcium or argon in Gates’s experiments). The resulting ions shoot down a pipeline into the cyclotron, which sweeps the ions around in a spiral, accelerating the beam.
Technicians in the control room use electrostatic forces to direct the beam out of the cyclotron and into instruments in the “caves,” low corridors that come off the cyclotron like spokes. The caves contain beam targets; the one in Cave 1 is a thin metal foil about the diameter of a salad plate. The targets rotate so the beam doesn’t hit any single spot for too long. They can melt when bombarded with speeding ions, Gates says.
What the target is made of depends on how many protons the researchers want in the final product. For example, to make flerovium (114 protons, named after Russian physicist Georgy Flerov, who founded JINR), they need to hit plutonium (94 protons) with calcium (20 protons). To make element 118, oganesson, scientists beam calcium at californium (98 protons). The more neutrons they can pack into the ion beam, the more they can ultimately cram into the final product, making even heavier isotopes.
Most of the time the beam passes right through the target without any nuclear interactions. But with six trillion beam particles winging through the targets per second, an eventual nucleus-to-nucleus collision is inevitable. When conditions are just right, these pileups mash the nuclei together, creating a very temporary new superheavy atom moving at nearly 600,000 meters per second.
Jen Christiansen
To slow down these speeding heavyweights, the researchers use helium gas and electric fields to guide the particles into a trap for measurement. They can also pump in other gases to see what kinds of chemical reactions a superheavy element will undergo before it decays. But that’s feasible only if the element lasts long enough, says Christoph E. Düllmann, head of the superheavy element chemistry research group at GSI. To conduct and study chemical reactions, researchers require an element with a half-life of at least half a second.
Scientists quantify superheavy elements and their reaction products by measuring the energy they give off during alpha decay, the shedding of bundles of two protons and two neutrons. In a room called the Shack at LBNL, researchers wait on tenterhooks for data points showing them where these alpha-decay particles land inside the detector; their journey reveals information about the composition of the original atoms and any reactions they’ve undergone. It’s hard to imagine that chemistry physically happening, Pore says: “It almost feels like it exists somewhere else.”
The heaviest element that researchers have studied chemically is flerovium (114)—the heaviest one that can be created in the quantities and with the duration needed for chemical experiments. Scientists can produce flerovium at a rate of about three atoms a day, Düllmann says. “A typical experiment needs about one month of total run time,” he says. “Not every atom that is produced will reach your chemistry setup, and not every atom that reaches your chemistry setup will be detected in the end.”
A few atoms can reveal a lot, however. Before flerovium was synthesized, some theories predicted that it might act like a noble gas—inert and nonreactive—and others suggested it might act like a metal, specifically, mercury. Experiments on the element published in 2022 in the journal Frontiers in Chemistry showed something weirder. At room temperature, flerovium forms a strong bond with gold, very unlike a noble gas. It also bonds with gold at liquid-nitrogen temperatures (–196 degrees Celsius). Oddly, though, at temperatures between these two, the element doesn’t react.
Oganesson is grouped in the periodic table with the noble gases, but researchers think it is neither noble nor a gas. It’s probably a solid at room temperature, according to research published in 2020 in Angewandte Chemie, and transitions to liquid around 52 degrees C. There are many such examples, says Peter Schwerdtfeger, a theoretical chemist at Massey University in New Zealand and senior author of the 2020 paper.
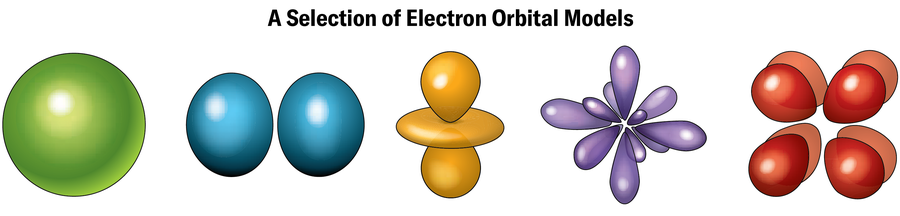
The reason for these strange characteristics has to do with the electrons. Electrons orbit nuclei at certain energy levels known as shells, each of which can hold a specific number of electrons. Electrons in outer shells—where there may not be enough electrons to completely fill the shell—are responsible for forging chemical bonds with other atoms. Each shell ostensibly represents a specific distance from the nucleus, although the actual path of an electron’s orbit in that shell (called an orbital) is often far from a simple circle and can look more like a dumbbell, doughnut, teardrop, or other configuration. (According to quantum mechanics, these outlines merely represent the places where an electron is likely to be found if pinned down by an actual measurement. Otherwise, electrons mostly exist in a haze of probability somewhere around the nucleus.)
Jen Christiansen
As a nucleus gets heavier, electrons near it feel an extreme pull from the glut of positive charges there, drawing them in closer and reducing the space they have to move around in. Because of the uncertainty principle, which states that a particle’s position and speed can’t be known precisely at the same time, this reduction in the electrons’ elbow room means their velocity must increase via a kind of seesawing of fundamental physical laws. Soon the electrons are traveling at nearly the speed of light. As Einstein’s special theory of relativity suggests, objects moving this fast gain mass and get weird.* In particular, the orbits of electrons in the lowest-energy states—the innermost shells—around a superheavy nucleus tend to contract, creating a greater density of electrons closer to the nucleus, Schwerdtfeger says. These changes are known as relativistic effects.
These effects show up even in naturally occurring elements of the periodic table. Gold is yellowish because relativistic effects shrink the gap between two of its electron shells, slightly shifting the wavelengths of light that the element absorbs and reflects. Yet relativistic effects don’t usually play a huge role in the chemical behavior of most light elements. That’s why the order of elements in the periodic table is based on the number of protons in each element’s nucleus. This arrangement serves to group together substances with similar chemical properties, which are determined mainly by the number of electrons in outer shells that are available for chemical bonds.
“The periodic table is supposed to tell you what the chemical trends are,” LBNL’s Pore says. For heavier elements, in which relativistic effects start to rule, that’s not necessarily true. In research published in 2018 in the journal Physical Review Letters, Schwerdtfeger and his colleagues found that because of relativistic effects, oganesson’s electron cloud looks like a big, fuzzy smear with no major distinction between the shells.
Even outside superheavy territory, chemists debate the placement of certain elements in the periodic table. Since 2015 a working group at the International Union of Pure and Applied Chemistry has been refereeing a debate over which elements should go in the third column of the table: lanthanum and actinium (elements 57 and 89) or lutetium and lawrencium (71 and 103). The debate centers on misbehaving electrons: because of relativistic effects, the outermost electrons orbiting these elements aren’t where they should be according to the periodic table. After nine years of official consideration, there is still no consensus on how to group these elements. Such problems only become more pressing at the heavier end of the table. “We’re trying to probe where that organization begins to break down and where the periodic table begins to stop being useful,” Gates says.
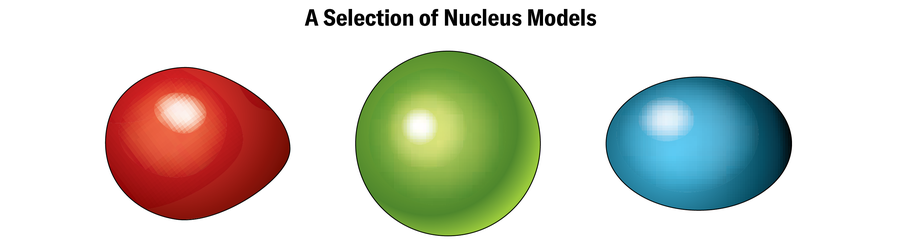
Along with a window into the limits of chemistry, the dance of electrons can provide a peek into the dynamics of the nucleus at the extremes. In a nucleus groaning with protons and neutrons, interactions between these particles often warp the shape into something other than the stereotypical sphere you’ll see in diagrams of atoms. Most of the superheavy elements that have been probed so far have oblong nuclei shaped like footballs, says Michael Block, a physicist at GSI. Theoretically, heavier ones that haven’t been synthesized yet might have nuclei shaped like flying saucers or even bubbles, with empty or low-density spots right in the center. Scientists “see” these shapes by measuring minuscule changes in electron orbits, which are affected by the arrangement of the positive charges in the nucleus. “This allows us to tell what the size of the nucleus is and what the shape of the nucleus is,” Block says.
Jen Christiansen
The layout of the nucleus holds the key to whether anyone will ever be able to synthesize a superheavy element that sticks around. Certain numbers of protons and neutrons (collectively dubbed nucleons) are known as magic numbers because nuclei with these numbers can hold together particularly well. Like electrons, nucleons occupy shells, and these magic numbers represent the tallies needed to fill nucleonic shells completely. The island of stability that researchers hope to find in a yet undiscovered superheavy element or isotope would be the result of “double magic”—theoretically ideal numbers of both protons and neutrons.
Whether such a thing exists is an open question because heavy nuclei might tear themselves apart rather than tolerating the required numbers of nucleons. “Fission is the killer,” M.S.U.’s Nazarewicz observes.
Unlike the (relatively) gradual whittling down of a nucleus by alpha decay, nuclear fission is a sudden and utter dissolution. Different models yield different predictions about how many particles can be packed into a nucleus before fission becomes inevitable, Nazarewicz says. Theorists are trying to determine this limit to understand how large nuclei can truly get.
There is an interesting liminal space at the edges of what nuclei can bear, Nazarewicz notes. To be declared an element, a nucleus must survive for at least 10–14 second, the time it takes for electrons to glom on and form an atom. But in theory, nuclear lifetimes can be as short as 10–21 second. In this infinitesimal gap, you might find nuclei without electron clouds, incapable of chemistry, he says.
“The periodic table breaks with the heaviest elements already,” Nazarewicz says. The question is, Where do you break chemistry altogether? Another way to understand superheavy elements is to look for them in space. The elements heavier than iron (atomic number 26) form in nature through a process called rapid neutron capture, which often occurs in cataclysmic events such as a collision of two neutron stars.
Jen Christiansen; Source: National Center for Biotechnology Information; https://pubchem.ncbi.nlm.nih.gov/periodic-table/ (reference)
If superheavies have ever arisen naturally in the universe, they were made by this process, too, says Gabriel Martínez-Pinedo, an astrophysicist at GSI. In rapid neutron capture, also known as the r-process, a seed nucleus grabs free nearby neutrons, quickly taking on the mass to make heavy isotopes. This must happen in an environment with ample neutrons roaming freely, which is why neutron star mergers are opportune spots.
In 2017 scientists observed a neutron star merger for the first time by detecting gravitational waves created by the interaction. “That was the very first confirmation that, indeed, the r-process happens during the merger of two neutron stars,” Martínez-Pinedo says. Researchers detected isotopes of lanthanide elements (atomic numbers 57 to 71) in that merger but, as they reported in Nature at the time, couldn’t narrow down the exact elements present. Detecting any superheavy elements will be even trickier because researchers will need to know which unique wavelengths of light those elements emit and absorb and pick them out of what Martínez-Pinedo calls the “complicated soup of elements” that emerges from one of these events.
In December 2023, however, astronomers reported in the journal Science that there are excess amounts of several lighter elements—ruthenium, rhodium, palladium and silver—in some stars. These elements may be overrepresented because they are the result of heavy or superheavy elements breaking apart via fission. The findings hint that nuclei with as many as 260 protons and neutrons might form via the r-process.
Even if superheavy elements created in neutron star mergers were to decay away quickly, knowing they existed would help scientists write a history of matter in the universe, Martínez-Pinedo says. New observatories such as the James Webb Space Telescope and the upcoming Vera C. Rubin Observatory in Chile should make it possible to see other cosmic events capable of creating superheavy elements. “And there will be new gravitational-wave detectors that will allow us to see much larger distances and with higher precision,” he adds.
At the Facility for Rare Isotope Beams in Michigan, a new high-energy beam promises to give further insights into the r-process by packing more neutrons into isotopes than ever before possible. These are not new superheavies but beefed-up versions of lighter elements. In February researchers reported in the journal Physical Review Letters that they had created heavy isotopes of thulium, ytterbium and lutetium using just one 270th of their beams’ ultimate planned power output. At higher power levels they should be able to make the kinds of isotopes that eventually decay into heavier stable metals such as gold. “This may provide a pathway to some of the interesting isotopes for astrophysics,” says Brad Sherrill, a physicist at M.S.U. and a co-author of that study.
Meanwhile other scientists around the world are also looking to amp up their ion beams and targets to push past element 118. In addition, they’re increasing the precision with which they can capture and measure these elements. Researchers at the Facility for Rare Isotope Beams plan to improve their ability to differentiate between particles by a factor of 10. GSI will soon have a next-generation accelerator for superheavy synthesis. And at LBNL, Gates and her team are installing instruments to take higher-precision measurements of the mass of single atoms.
These new tools should further reveal the contours of chemistry at the extremes. “When we do superheavy chemistry,” Massey’s Schwerdtfeger says, “we see surprises all over the place.”
*Editor’s Note (7/9/24): This sentence was edited after posting to correct the reference to Einstein’s special theory of relativity.

