Kobus Steenkamp’s farm sprawls along a dirt road in South Africa’s central plains, where the sky makes everything seem small. Steenkamp woke up here one morning after the rains in 2010 to find something strange happening with his sheep. “You could see there’s blood at their backs,” he recalls. All his pregnant ewes were losing their lambs.
It was every farmer’s nightmare: his herd had been infected by Rift Valley fever, a mosquito-borne virus that causes abortion and death in livestock and wildlife and can be transmitted to humans. Within days, dozens of people had also been infected. Most displayed only flulike symptoms, but in some cases, the illness escalated into a severe hemorrhagic fever akin to Ebola.
A similar scene was unfolding across the region. The survival rate in adult animals is as low as 10 percent, and nearly 100 percent of infected sheep abort their pregnancies. Dead lambs and calves were left bloating in fields until the state veterinary team came to collect and incinerate the carcasses. By the time the outbreak was under control, almost 9,000 animals and 25 people had died. Neighboring countries, such as Zimbabwe and Namibia, banned South African meat, shattering the livestock industry.
On supporting science journalism
If you're enjoying this article, consider supporting our award-winning journalism by subscribing. By purchasing a subscription you are helping to ensure the future of impactful stories about the discoveries and ideas shaping our world today.
Ever since the virus was first identified in 1931 in Kenya’s Rift Valley, outbreaks like this one had been confined to southern and eastern Africa. But in 1977 the disease migrated north through increased trade on the Nile River, causing what the World Health Organization called an “explosive outbreak” in Egypt. Then, in September of 2000, it jumped to the Arabian Peninsula, arriving in Saudi Arabia and Yemen—sparking anxiety that Europe and North America were next. The idea that the virus could spread across these continents in just a few years is not some hyperbolic scenario. Rift is transmitted through a broader range of hosts and vectors than West Nile virus, which arrived in New York City in 1999 and spread across the U.S. in less than six years. The U.S. Department of Agriculture has taken notice, naming Rift the third most dangerous animal pathogen, behind only bird flu and foot-and-mouth disease. But health officials are not just worried about its impact on animals and agriculture. Zoonotic diseases—infectious illnesses such as Rift that begin in animal populations and jump to humans—are the biggest risk for epidemics and pandemics. They have been responsible for some of history’s worst, including bubonic plague and Ebola.
The fear of Rift growing into a global pandemic highlights that public health researchers still don’t know how to effectively predict disease outbreaks, which have devastating consequences on health, economies and political stability. Meanwhile the threat of emerging zoonotic diseases is expanding—and often in unforeseen ways. Scientists are only just beginning to understand how outbreaks correlate with shifting weather patterns—a hallmark of climate change. As they do, the picture is becoming more complicated. Worldwide, temperatures are changing faster than anyone previously predicted, and as a result, habitats are, too—altering the ranges of animals, viruses and, increasingly, humans. These complex relationships are now more volatile than ever, leading one recent Lancet paper to conclude that climate change is “the biggest global health threat in the 21st century” and yet another in the Lancet to suggest that it “threatens to undermine the last half century of gains in development and global health.”
Global warming and extreme weather patterns are already dramatic forces on public health. Intensified floods, droughts and storms are changing how humans can use land and, ultimately, where we can live. As climatologists race to model what has changed so that coastal communities, for instance, can adapt to rising seas, epidemiologists are also realizing how critical it is to develop epidemic-prediction tools that incorporate new and impending weather patterns. Such research is a matter of equity between developing and developed nations. And in an ever more globalized world, it may be a way of averting a future of unprecedented pandemics.
An Interconnected Approach
To get to Steenkamp’s farm, biologist Ettienne Theron has been driving for hours toward an endless horizon. His truck, loaded with coolers full of blood, bounces down a crumbling highway that is flanked by open grassland. These velds are where the last several Rift epidemics in South Africa began. It’s here, in an area larger than the size of Maryland, that Theron and dozens more researchers are collecting and analyzing data for a project run by EcoHealth Alliance, a global nonprofit that focuses on pandemic prevention. [Editor’s Note: This story was reported before the COVID pandemic drew more attention to EcoHealth Alliance, which was a partner with the Wuhan Institute of Virology as part of its work around the world.]
The challenge for scientists and policy makers alike is to learn how to intervene before pathogens infect people. Once a pandemic emerges, said EcoHealth president Peter Daszak in a 2015 video, “all you can really do is put out the fire.” The goal of this five-year, multidisciplinary project is to examine, for the first time, exactly how climate affects Rift Valley fever in southern Africa. In doing so, researchers hope to develop a data-based model that actually predicts outbreaks before they happen, a stepping-stone to making models for other viruses as well. Notably, the U.S. Department of Defense has funded the entire project. Rift can easily be used as an aerosol and was weaponized by both the U.S. and the Soviet Union during the cold war. But bioterrorism is only one concern; keeping diseases from reaching the U.S.’s shores—and knowing how to react if they do—is increasingly a matter of national security.

At one of 22 weather stations set up by the EcoHealth Alliance's Rift Valley fever project, Zikhona Gqalaqha, a graduate student at the University of the Free State in South Africa, collects data on soil moisture. Credit: Sean McDermott
Theron finally arrives at an unmarked gate, and the team members, who collectively speak nine of the languages represented in the Free State province, pile out of the truck and pull on muck boots. Using a random distribution of GPS points, the researchers have reached an agreement with 361 isolated farms like Steenkamp’s, where, for two years in a row, they have taken blood samples from livestock and farm staff to test for Rift antibodies, trying to understand where the virus might be lurking when no one is reporting active cases.
Steenkamp himself was infected during an outbreak in the 1970s, and according to the WHO, as many as 10,000 people a year contract the virus. That number will most likely rise. A 2016 study published in Emerging Microbes & Infections reports that the “explosive nature” of recent epidemics suggests the virus has mutated into a more infectious and severe strain. As it migrates to new places, the virus may evolve further, becoming even more dangerous.
As the farm crew maneuvers sheep into a corner of a rusted corral, field coordinator Claudia Cordel unpacks an arrangement of empty vials, data sheets, latex gloves and a sharps box on a folding card table. A farm worker grabs an ewe. It bleats while Cordel draws blood from its jerking neck. In South Africa, Aedes mcintoshi mosquitoes are thought to be the main carrier of Rift. The females transmit the virus directly to offspring, and their eggs can survive years of drought—a typical occurrence in this region. When the new generation of infected mosquitoes eventually hatch, they transmit the virus to livestock and wildlife alike. The virus amplifies in these hosts, so that when wider-ranging mosquitoes, such as Culex and Anopheles, bite infected animals, the original outbreak transforms into a swift-moving epidemic.
In another corral, Cordel wipes a cow’s anus before piercing its tail vein. The cow lows and lets loose a squirt of green liquid toward her face. Cordel explains that though the basics of the virus transmission are known, “we have no idea how wildlife impacts people or livestock, or vice versa,” adding that the feedback cycles are completely undocumented. That is why the EcoHealth team wants to get a more granular look at the interactions of weather, plants, insects, animals and people. At 22 research sites around the Free State and Northern Cape provinces, researchers trapped mosquitoes to search for the virus, studying soil composition and vegetation and setting up mini weather stations to monitor local conditions in conjunction with satellite data. This kind of comprehensive approach, which required dozens of experts in epidemiology, ecology, climatology, veterinary medicine and entomology, is both costly and relatively rare. But it may be the future of understanding how infectious diseases emerge and spread.
“It makes sense that the health of an animal population is related to the health of the human population,” says Melinda Rostal, one of the project’s investigators. Animals often serve as early-warning signs of a new outbreak; in 2017 in Brazil, for example, local monkey populations were nearly wiped out eight months before a yellow fever epidemic. But structuring research around interactions among people, animals and the environment has only recently gained traction in the global health community. This strategy, first defined by epidemiologist Calvin Schwabe in 1964 and now called “One Health,” is an increasingly popular intellectual framework for epidemiology. As far back as 400 B.C., Hippocrates understood that the environment—including weather—impacts disease, but systematically bringing together multidisciplinary research to better understand complex systems is relatively new. The U.S. Centers for Disease Control and Prevention did not establish a One Health office until 2009, when officials acknowledged that changing environmental interactions “have led to the emergence and reemergence of many diseases.” Pursuing One Health research is expensive up front, but in the long run, it can actually be more efficient: by sending out collaborative teams instead of funding individual research trips, the EcoHealth Rift project reduced the cost of transportation for its study by 35 percent.
The longtime absence of this style of coordination is partly why the global health community is still playing catchup on emerging diseases. Consider Zika, for example: although it was first identified in Uganda in 1947, it was largely ignored until it began tearing through the Americas in 2015. Such diseases often lack attention when they first emerge because they affect the poorest populations of the world, meaning they are generally not profitable for pharmaceutical development. The result is that these so-called neglected tropical diseases, according to the CDC, have already cost 57 million years of life lost prematurely. So as one of the largest One Health projects to date, EcoHealth’s Rift work is an important case study: Can broad, multidisciplinary research projects fill this dangerous knowledge gap?
As the setting sun turns the grass gold, Cordel and Theron finish the farm visit by checking on a weather station, where a lonely wind propeller ticks above barbed wire. Satellite data from NASA suggest that this region will see altered weather patterns, changing its risk of both Rift and other infectious diseases. Transmission is a complicated thing, but undoubtedly a key factor in future disease control will be understanding the implications of our changing climate.
Forecasting Climate on a Backyard Scale
On the long drives between field sites, dead sunflowers droop under a relentless sky. South Africa has been in a drought for a few years, and the red soil has frazzled into puzzle pieces. Drought itself is a standard feature of El Niño weather patterns, and when La Niña eventually comes and completes the cycle, the area will see heavy rains. But these cycles, while typical, are intensifying because of climate change, becoming drier and wetter, explains Assaf Anyamba, a research scientist at the NASA Goddard Space Flight Center. (In March 2018 South Africa declared a “national state of disaster” over its prolonged drought. Cape Town was at risk of running out of water during the worst drought in 400 years.) Even as conditions grow more extreme, Anyamba says, the downpour associated with this rain pattern—known as the El Niño/Southern Oscillation (ENSO)—and the vegetable life that downpour creates are what makes it possible to predict the hatching of Rift-infected mosquitoes. In fact, Anyamba was able to successfully predict the 2006 and 2007 Rift outbreak in East Africa by adding satellite climate models to the mix instead of just relying on regional weather patterns. “To my knowledge,” he says, “this is the only system of its kind for any disease.”
With that promising model in hand, Anyamba looked to southern Africa and the Arabian Peninsula. If he could apply the tools he used in eastern Africa to predict Rift outbreaks elsewhere, perhaps he could expand the model to other diseases. But so far his Rift models have failed in South Africa. As the climate expert on the EcoHealth study, Anyamba is now trying to figure out why. Satellite data that show and forecast global weather patterns make it easier to predict changes with vegetation and insects. The downside is that this big-picture view is fairly imprecise. When researchers combine climate models with more granular regional data such as vegetation coverage, they are dealing with two different scales. Anyamba’s eastern African satellite models relied on a vegetation index, for example, that did not reflect southern African plant species. Other factors that impact disease, such as the spread of vectors, can be even finer-grained. Many mosquitoes live in an area the size of a suburban backyard, so even remotely sensed data do not get at the scale with which pathogens interact with their hosts. Although weather has long been linked with disease—think “flu season”—it is this level of specificity that makes predicting outbreaks so challenging. A one-size-fits-all template will not work.
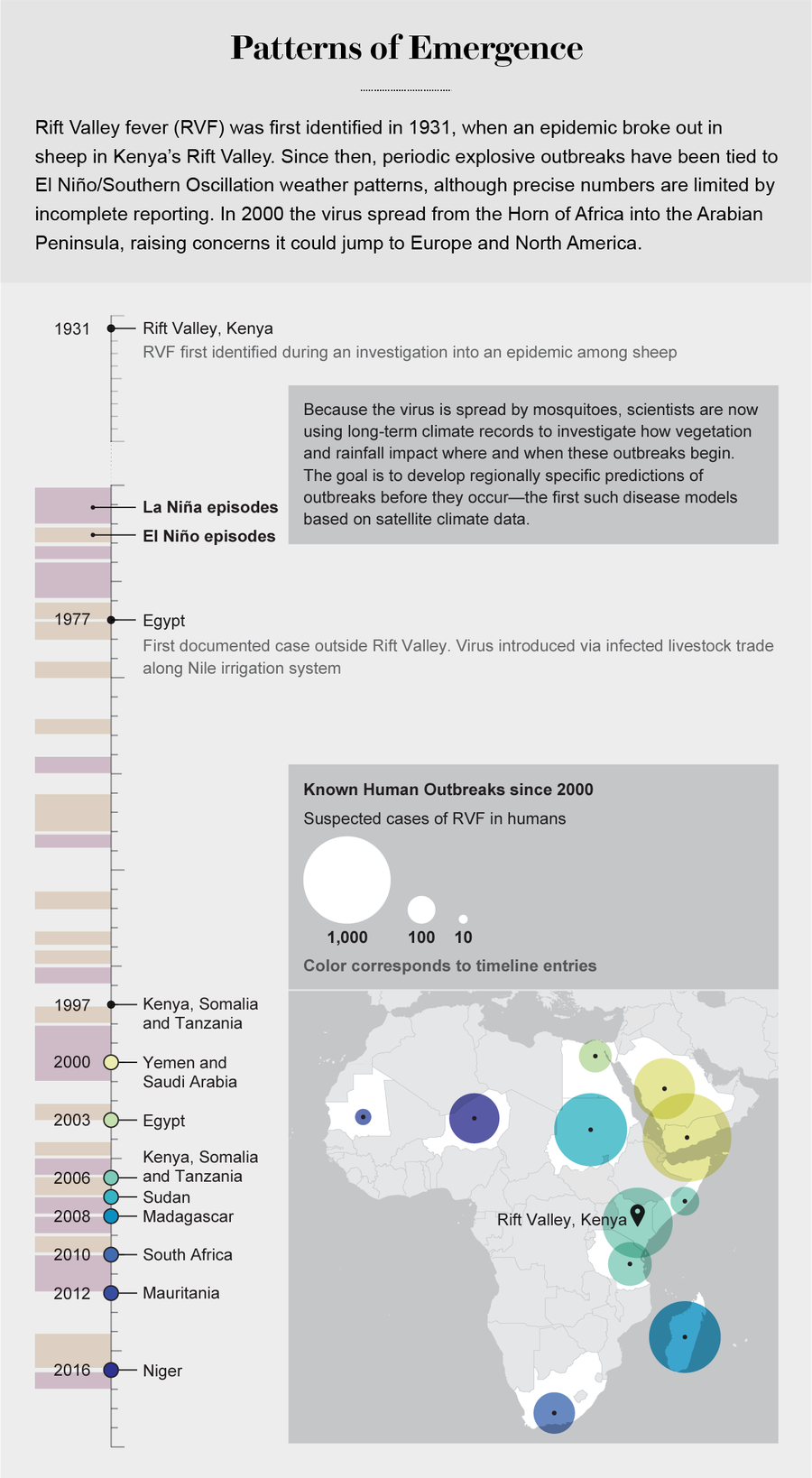
Credit: Tiffany Farrant-Gonzalez; Sources: World Health Organization and Centers for Disease Control and Prevention (outbreak data); National Weather Service (El Niño and La Niña data)
Anyamba’s new tactic is to use the information the EcoHealth team gathers on mosquitoes and vegetation in South Africa to build a more customized prediction model for the region. Climate change may eventually make the Free State province drier, which would help prevent Rift outbreaks. Other parts of the country, however, will likely get warmer and wetter, increasing Rift and the other diseases that floods tend to foster. Learning how to build more regionally sensitive tools will help scientists understand how disease burdens may change, both locally and globally.
Getting there is a matter of urgency. While it is rare to find sweeping conclusions in epidemiology, it is clear that greater climate variability—and therefore greater disease fluctuation—is already here. The first conclusive evidence of the trend was likely initially reported in a 2002 study published in the Proceedings of the National Academy of Sciences USA, which looked at cholera prevalence in Bangladesh over a 70-year period and found that “warming trends over the last century are affecting human disease.” Mosquito and other insect habitats have expanded because of warming, exposing new populations to viruses. Preliminary research shows that malaria, for example, is globally on the rise. A temperature bump of just two degrees Celsius—a mark we are quickly approaching—would expand the number of people at risk of malaria by several hundred million, according to the WHO. Strangely, places that are now ideal climates for malaria may see less of it as they warm; prevalence will likely occur where malaria has not yet arrived, such as the U.S.
This type of vexing nuance has troubling consequences. Take bluetongue virus, a highly lethal ruminant disease that is spread by biting midges called Culicoides. Historically it was confined to tropical regions, but by 2006 there was enough warming in western Europe that some of those midges moved north and infected animals. Scientists were surprised when another kind of midge then picked up the virus from sickened sheep and carried it all the way to Norway. Corrie Brown, a veterinary pathologist at the University of Georgia, says bluetongue is a prime example of how climate change introduces species to one another for the first time—expanding how diseases can spread in an unpredictable manner.
Experts disagree on the best way to handle these risks. The U.S. Agency for International Development supports a strategy that focuses on identifying new pathogens, but Brown thinks merely discovering new viruses is an inefficient use of limited research funding. “I can see how very good it’d be for the investigators because they’d get a lot of papers published,” Brown says, but she is bearish on its value in preventing people from getting sick. Instead Brown and others who advocate for a One Health approach think strengthening local infrastructure—building monitoring and surveillance systems and training community nurses, for instance—is the most effective way to grapple with the fickle burdens of emerging diseases. “If we improve the level of expertise of health-care professionals all around the world, we’ll be in a better place,” she says.
Local detection systems are especially important in places where humans are the ones who have moved into new environments, exposing themselves to diseases they have not yet encountered. “In an unchanging world, you don’t see a lot of emerging disease,” says William Karesh, an epidemiologist and the Rift study’s principal investigator. “It’s when systems alter that microbes reveal themselves in new ways.” Often epidemics and their ripple effects “happen at the edge, where humans are living next to wild spaces,” says Carrie La Jeunesse, a former AAAS Congressional Science & Technology Policy Fellow who worked on Ebola. Since 2009 USAID has developed a heat map for emerging diseases with pandemic potential; it is remarkably similar to maps of regions threatened by human impact. In a 2012 paper published in the Lancet, Karesh and his colleagues summarized these findings by explaining that “many zoonoses can be linked to large-scale changes in land use.”
That is certainly true in South Africa. “We actually farm with arboviruses,” the viruses transmitted by arthropods such as ticks, says Alan Kemp, an entomologist on the EcoHealth project. “With Rift, it’s almost certain that thanks to cattle breeding and importing exotic breeds that aren’t resistant, we’re actually literally farming Rift.” He sighs and says, “To be honest, to a large extent, we’re guilty of our own demise.”
The Human Strain
The thousands of blood samples EcoHealth has procured end up under the fluorescent gleam of a biosafety level–four laboratory in Johannesburg. Like Ebola, work with Rift is allowed only in the highest level of containment, and investigator Janusz Paweska wears a pressurized protective suit to examine Rift specimens under a microscope. “Some scientists refer to nature as the most terrible bioterrorist, which I dislike,” says Paweska from his office, after he has been through the elaborate decontamination process. As head of South Africa’s Center for Emerging Zoonotic and Parasitic Diseases, he does not mince words: “Who creates this environment for emergence? You can’t accuse nature. Uncontrolled urbanization, climate change, poverty—that’s not nature. The answer is that we create the situation for the emergence of many of these diseases.”
Arguably poverty is already the greatest risk factor for getting sick. “The major trigger or determinant of health is economic,” says Antoine Flahault, director of the Institute of Global Health in Geneva, explaining that an unequal distribution of health care is the primary problem. The WHO estimates that in low-income countries, diseases of poverty that are often preventable or treatable (think diarrhea, malnutrition and parasitic infections) account for 45 percent of deaths. Climate change is expected to drive at least 122 million people into extreme poverty in the next few decades, forcing many to leave their homes and leading to rapid urbanization, all of which tends to foster disease. Flahault expects that one of the major disease contributions of climate change will come from these consequences of forced migration. “We can expect a huge impact on health, not just because of direct impact on disease but because of the indirect economic impacts, which may be very severe,” Flahault says.
But when countries with limited resources are asked to prepare for a future potential threat, often at the cost of immediate problems, “it’s a hard trade-off,” explains Susan Scribner, director of the Preparedness & Response project at the global development firm DAI. “What we do is called health, but in some ways, a lot of it has to do with good governance,” she says. That is why projects such as the Rift study could be particularly powerful: they provide data to many different stakeholders spanning agriculture, health and defense.

Field team members take blood samples from farm workers and livestock to test for Rift Valley fever antibodies. Researchers are trying to understand how the virus is maintained between outbreaks. Credit: Sean McDermott
Anyamba, EcoHealth’s climate expert, sees the Rift study as the way of the future. “I envision more projects involving climate data, fused with advanced analytics and machine-learning technologies, that will begin to answer some questions [about] why particular disease outbreaks occur,” he says. Policy makers may be juggling other priorities, but it is important that they understand this science, Scribner says, “because when a pandemic hits, scientists aren’t the ones in charge of the response.” In fact, the DOD’s Defense Threat Reduction Agency funded similarly comprehensive research to predict and map areas at risk for chikungunya, another mosquito-borne viral infection. There is a long list of diseases, such as yellow fever, dengue and even rabies, that would benefit from the kind of resources the DOD can bring to bear.
But funding—and the politics that go into deciding whose research gets it—plays a critical role in which diseases are deemed worthy of attention. In the current political climate, support for the long-term resources that logistically complex projects (such as the Rift study) require is actively disappearing. Just as we are beginning to realize how urgent a collaborative approach to disease might be, the CDC has faced massive threats to its global health security efforts. Funding global health is a complex endeavor managed by multiple agencies in the U.S. alone. Experts at the Brookings Institution think tank have written that it could be “devastating for global health,” damaging economic growth as well as weakening international stability.
But the importance of doing this kind of intensive fieldwork continues. Amid this limbo, EcoHealth’s sweeping fieldwork presses on. Early one morning during its collection process, the team pulls up to a private game reserve near Mokala National Park to chase down kudu, a type of African antelope. Wildlife can both carry and transmit Rift to livestock and humans, but their blood samples are especially tricky to obtain. A helicopter arrives to lend a hand. Soon trucks are bouncing over the grass, a cloud of dust marking progress toward the herd. Beyond the front tires, the animals swerve and leap. They gleam like ribbons. The stillness when one is tranquilized from the sky is shocking. The scientists work quickly, sliding around a maze of arms and legs with syringes and vials. The kudu will wake in a matter of minutes.
After the blood is collected, the researchers begin their decontamination procedures, scrubbing dust off their boots to avoid spreading any diseases to the next property they visit. Nearby, a caged lion lounges in a puddle of sunshine, awaiting the delivery of his next meal. He yawns at the stream of passing cars. Once this bushveld stretched wild over half of the country, but today it has been largely partitioned off and contained behind tall fences. There are few places now where wildlife are still able to wander free. The world has already changed, even if we do not yet know the consequences.

