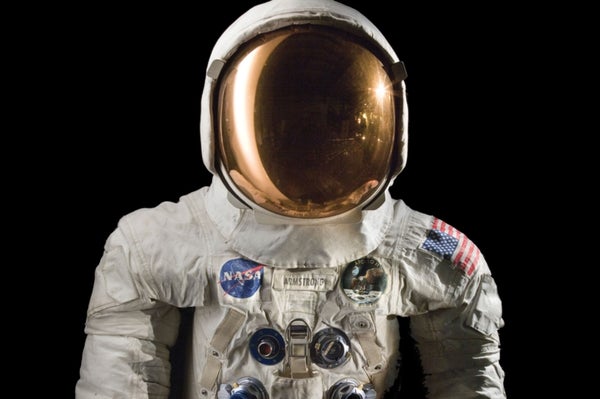
These men's suits were built to last. They were pristine-white and composed of 20-plus layers of cutting-edge materials handcrafted into a 180-pound frame of armor. They protected the wearers from temperatures that fluctuated between −300 and 300 degrees Fahrenheit and from low atmospheric pressure that could boil away someone's blood. On a July day in 1969, the world watched intently as astronaut Neil Armstrong, wearing one of these garments, stepped off a ladder and onto a dusty, alien terrain, forever changing the landscape both of the moon and of human history. Few symbols of vision and achievement are more powerful than the Apollo mission spacesuits.
Back on Earth, the iconic garments found new lives as museum pieces, drawing millions to see them at the National Air and Space Museum in Washington, D.C. And staff members there have found, to their surprise, that the suits need their own life support. They are falling apart.
Last year Lisa Young, a conservator at the museum, noticed that a white, foggy bloom was beginning to creep across the transparent fishbowl helmets and that their smooth, curved surface was beginning to crack. “It is really frustrating,” Young says. “We had thought they were relatively stable.” There had been warning signs of suit trouble, though. The neoprene pressure bladders that kept astronauts' bodies from exploding in the vacuum of space began crumbling years ago, releasing acidic gases. “Anybody who has worked with the spacesuits knows their smell,” Young says. “I'd describe it as slightly pungent sweet chlorine.” And an orange-brown sticky stain began appearing on the exterior white fabric.
On supporting science journalism
If you're enjoying this article, consider supporting our award-winning journalism by subscribing. By purchasing a subscription you are helping to ensure the future of impactful stories about the discoveries and ideas shaping our world today.
The trouble is the construction material: plastic. Most people think plastics last forever, which makes them a bane to the environment. But although the repeating units of carbon, oxygen, hydrogen and other elements in plastics have a long lifetime, the overall chains—synthetic polymers—do not age well. Light conspires with oxygen and temperature to weaken the bonds that hold the units together. Then chemicals added to plastics to make them bendable or colorful migrate outward, making the surface sticky and wet and perfect for attracting dirt. The polycarbonate spacesuit visor, Young thinks, was leaching out a substance added to make it easier to shape.
Priceless 20th-century art is in serious trouble as well. In that era, Andy Warhol, David Hockney and Mark Rothko all used acrylic paint—a plastic polymer popularized in the 1940s as an alternative to traditional oil paint. Plastic is, in fact, a building block of much of our recent cultural heritage, including important designer furniture, archival film, crash test dummies, the world's first Lego pieces and Bakelite jewelry, as well as the plastic sculptures made by the pop-art movement. “We now know that objects made of plastic are some of the most vulnerable in museum and gallery collections,” says Yvonne Shashoua, a conservation scientist at the National Museum of Denmark and one of the first cultural heritage researchers to study plastic degradation.
The conservation field is now racing against time, trying to keep pace with the material's unexpectedly rapid deterioration. Conservators have identified the most trouble-prone plastics. Scientists are developing new tools to diagnose plastic degradation before it becomes visible to the human eye—for example, by measuring the molecules wafting off artifacts. Researchers are also devising new strategies for freshening up precious plastic art without harming it, using everything from cleaning solutions called microemulsions to polyester microfibers that gently remove dirt.
Degrading Denial
The realization that plastics were a problem dawned slowly. For most of the 20th century the museum world was afflicted with “plastics denial syndrome,” Shashoua says. “Nobody thought that plastic objects in their collections would degrade.” In fact, some conservators were so enamored with plastic during its heyday of the 1950s, 1960s and 1970s that they used the polymers in ill-advised ways themselves. For example, conservators laminated Belgium's oldest parchment, the Codex Eyckensis from the eighth century a.d., with PVC plastic for protection. Decades later this laminate had to be painstakingly separated from the parchment because changes in the PVC began exacerbating the ancient document's demise.
Crash test dummies first made Shashoua think plastic was not forever. She had grown up visiting London's Science Museum, where dummies built in the 1970s to better understand the human toll of automobile collisions were on display. The mock bodies—among the first of their kind—have a metal frame skeleton enveloped by medical gelatin that has been sculpted into human form and then covered by a layer of protective PVC. During impact tests, encapsulated red paint would bleed out of the gelatin bodies and get caught underneath the PVC layer wherever the dummy had smashed against a car frame during collision experiments. The red wounds indicated the body's most vulnerable regions.
As the decades passed, these same crash test dummies in the museum began bleeding again. Shashoua was shocked to see that the PVC covering these artifacts was collapsing, dripping so much wet, sticky muck that museum staff had set up petri dishes in the showcase to collect the mess. When Shashoua was put in charge of cleaning the artifacts in 2011, she noticed that the dummies' sculpted contours were losing their definition as the PVC plastic collapsed; in some parts, the red paint mixed with the wounded plastic, giving the goo dripping from the dummies an eerily realistic brownish-red tinge.
This dripping mess—and in fact, all kinds of plastic degradation—owes its start to oxygen. With help from light and heat, the gas rips off the electrons from the long polymer chains that entwine to form a plastic object. Losing electrons can weaken and break chemical bonds in a plastic, undermining its structure. Essentially the long chains break up into smaller constituent molecules called monomers. In the case of the crash test dummies, this destabilization allowed ingredients called plasticizers, which are added to make the plastic supple, to pour out.
When the museum world began to realize that plastics were not invincible to time, those tasked with protecting plastic art and artifacts had to start from scratch to understand in detail why their collections were breaking down, says Matija Strlič, a conservation scientist at the Institute for Sustainable Heritage at University College London. Although there was extensive literature on polymer production, this research stopped at the end of a plastic object's expected lifetime—right when conservators get interested, Strlič says. Polymer makers had probably expected that old plastic objects would get tossed away, not delivered to museums.
The Feared Four
Conservators learned that four kinds of plastic polymers are especially prone to problems: PVC, found in everything from spacesuit life-support tubing to crash test dummies; polyurethane, a primary ingredient in products as diverse as panty hose and packing sponges, as well as sculptures made from these materials; and finally cellulose nitrate and cellulose acetate, two of the world's first industrially produced synthetic polymers, found in the film used in early cinema and photography, as well as in artificial tortoiseshell items, such as vintage combs and cigarette holders.
Cellulose acetate and cellulose nitrate are not only fragile, they are also often referred to as “malignant” by conservators, Shashoua says. That is because they spread destruction to nearby objects. As their polymer networks collapse, they release nitric acid and acetic acid as gases. (Acetic acid is what gives vinegar its characteristic smell and degrading film an odor reminiscent of salad dressing.) The acids eat away at objects made of these plastics. To make matters worse, their gases can also corrode metal and textile things in the same display case or nearby storage. That smell of vinegar is not just an alarm bell that these objects are destroying themselves but that the degrading polymer is taking down innocent bystanders as well.
Shashoua has seen fashion display cases where the acids from a degrading plastic comb have begun eating away textile outfits showcased with the comb or where the plastic in faux tortoiseshell eyeglass frames releases acid that corrodes the spectacles' metal hinges. Once, in her own work space, a box containing knives with cellulose nitrate handles began releasing nitric acid that corroded both the metal blades and the hinges of a cupboard near where the utensils were being stored, Shashoua says. To stop these chemical attacks, conservators may put objects made of cellulose acetate in well-ventilated spaces to whisk away the dangerous gases. They also capture the poisonous gases in the tiny pores of filters made from activated carbon and zeolite, in much the same way gas masks protect troops exposed to chemical weapons.
Ventilation and trapping are good strategies against cellulose acetate and cellulose nitrate, but the methods do not work on all plastics, Shashoua says. For example, when PVC breaks down, if its degradation products are pulled away from the surrounding environment, the plastic just releases more. Instead conservators need to keep PVC locked down, sealed in airtight containers, to stall its demise. When conservators noticed that the pristine-white Apollo mission spacesuits were getting orangey-brown stains on their nylon exterior, they realized the cause was plasticizer leaching out of life-support tubing made of PVC that had been sewn into the textile. The tubing kept astronauts' bodies from overheating by circulating cooled water around the outfit. “We had to carefully remove all the life-support tubing from all the Apollo suits and store it separately in sealed containers,” Young says. “That was a lot of work.”
These opposing approaches—sealed containers versus ventilated ones—highlight why there is no one-size-fits-all solution. “No two objects are alike,” Strlič says. For this reason, conservation scientists try to identify the base polymer in a plastic artwork or artifact, typically with analytical machines such as a Fourier transform infrared spectrometer, which bounces long wavelengths of light off an object to reveal its unique molecular fingerprint. Conservators at the Solomon R. Guggenheim Museum in New York City used such a method to uncover a hidden danger in artwork by Bauhaus pioneer László Moholy-Nagy. They had believed the base material for his painting Tp2 was Bakelite (a phenol-formaldehyde resin), says Carol Stringari, head of conservation at the museum. But recent infrared spectrometer analysis by scientists affiliated with the Art Institute of Chicago revealed that the polymer was actually cellulose nitrate, one of the plastics that can release harmful gaseous acids.
Spectrometry used in this way is helpful, but it has limits. It can identify many ingredients, but it does not always show the entire potpourri of dyes, stabilizers, surfactants, plasticizers and antioxidants that are mixed into plastics. Often industrial manufacturers keep these recipes secret as part of their intellectual property. Because there is no easy reference for their components, it requires arduous analysis to uncover the plastic's chemical makeup.
These additives change the way an object will age and fall apart. Some varieties of PVC, such as the kind in the spacesuit's life-support system, break down by leaching a sticky plasticizer called di(2-ethylhexyl)phthalate. Other PVC objects degrade by developing a white, powdery crust on the surface: in this case, stearic acid is to blame. It is a lubricant added to the plastic to prevent the polymer from sticking to its mold during the manufacturing process.
Sniffing Out Decay
It is so important to identify the chemical mélange before developing a life-extension strategy that researchers are literally sniffing out the ingredients in plastic artifacts. For example, in a project aptly named “Heritage Smells,” Katherine Curran of University College London capitalized on the fact that a lot of degrading plastics emit stinky molecules. Not only does cellulose acetate smell like vinegar as it breaks down and aging neoprene like sickly sweet chlorine, but many other plastics also release volatile molecules as they disintegrate: degrading PVC has the aroma of a new car, and degrading polyurethane can smell like raspberry jam, cinnamon or burning rubber. These are just the odors detectable by the human nose. Curran developed a mass spectrometry technique that analyzes all the volatile molecules rising off plastic objects to pinpoint the additives and stabilizers breaking down in a plastic. The goal is to identify what is going on inside without needing to take a sample and to do so before there are visible signs of decay, Curran says.
Curran took her technique to the Birmingham Museum & Art Gallery, where she sampled the air around an enormous art installation made in 2005 by Benin artist Romuald Hazoumé called ARTicle 14, Débrouille-Toi, Toi-Même! which translates to ARTicle 14, Straighten Yourself Out, by Yourself! It features a market cart full to the brim with sports shoes, computers, a film reel, golf clubs, old Nokia phones, toys, pots, pans, high-heeled pink shoes and a vacuum cleaner, to name just a few components in the piece, which Hazoumé put together from objects he had collected during the 1990s and 2000s. Amid the chaotic artwork, Curran and her colleagues detected the presence of acetic acid, one of those corrosive gases that can hurt nearby materials. “We found that the film reel—specifically, degrading polyester in the film—was emitting the acid,” Curran says. Museum staff are now considering whether to store the film reel separately or use absorbents for the acid to prevent it from having a detrimental effect on other components of the piece, she says.
Curran has also tried out her canary-in-a-coal-mine technique at the Museum of London on a collection of vintage handbags—purses made of faux leather, mock tortoiseshell, or coiled, 20th-century telephone cords. In the case of the white-telephone-cord purse, Curran sniffed out the presence of plasticizers that typically emerge from degrading PVC—a useful alarm bell for staff who may want to store the purse in a sealed container.
Researchers are also turning to new imaging technologies that create detailed two-dimensional maps of the chemical composition of an object, essentially going pixel by adjoining pixel. For example, Strlič has combined near-infrared spectroscopy with a digital camera to produce two-dimensional colored maps from which conservators can identify the molecular makeup of artifacts that contain many types of plastic, as well as the migration of degradation chemicals. Strlič has gazed inside a popular vintage piece from the 1950s called a crinoline lady—where a plastic bust of a woman forms the handle of a hairbrush. Strlič's team used the technique to identify the handle as cellulose acetate and the brush hair as nylon, using color gradients to show the location of the two plastics in the artifact. By identifying potential dangers such as the acetate, museum staff might be able to take action before damage is visible to the naked eye.
Although researchers are getting better at diagnosing how a plastic artifact or artwork is degrading, they are still trying to figure out how to best stop the decay and repair damage. That was one challenge tackled by a project called POPART, or the Preservation of Plastic ARTefacts in Museum Collections, which started in 2008 and combines efforts from institutions around the world. Cleaning may make the object look better, but it might eventually accelerate the overall demise. A white crust on the surface might be unsightly but is also a protective patina, similar to the green oxidized layer that forms over aged copper as both a degradation product and a protective skin.
Cleaning Up
Even if washing off this patina is the right strategy, POPART researchers want cleaning methods that can do so safely. Conservators are very cautious—a good characteristic in those charged with caring for million-dollar art. And plastics can get cracked, dissolved or discolored when exposed to the wrong cleaning agent. POPART investigated approaches ranging from high-tech microfibers and ultrasound to carefully formulated cleaning microemulsions (solutions of water, oil and a surfactant that lifts dirt), as well as gels. The scientists learned that cleaning a polystyrene object with acetone—often used in nail polish remover—could turn the plastic from transparent to opaque and eventually dissolve it. Isopropanol, a different alcohol-based cleaning solvent, however, is safe for most plastics.
Using something as simple as water to clean acrylic paintings turns out to be risky, says Bronwyn Ormsby, a conservation scientist at the Tate, a group of four museums in England. She confronted that problem with the 1962 painting Andromeda, the Tate's oldest acrylic piece. Russian-American artist Alexander Liberman painted this abstract, geometric work on a circular canvas; its four solid colors—black, lilac, dark purple and dark green—evoke the darkness of outer space. But acrylic paints have additives called surfactants that help to keep pigments suspended in the paint tube rather than settling to the bottom. That is good for the painter. Yet once on a dried canvas, these surfactants migrate to the surface and create a sticky substance that attracts dirt. By 2007 Andromeda was obscured by so much surfactant buildup that the painting had “a whitish bloom, which is quite distracting on paintings with dark colors,” Ormsby says. Ordinarily, she would turn to water as a cleaner: “Water often removes soil better than any other solvent.” But water also makes acrylic paintings swell. That can lead to a loss of paint during the cleaning process.
Water can be tweaked to make it safer, though. Investigators led by Richard Wolbers of the University of Delaware have found that keeping water's pH levels around 6 and making the water moderately salty can limit the swelling of acrylic paint. Ormsby used that technique on the Liberman painting, which today looks as dark and lonely as it did five decades ago. Researchers at the Tate have also used an atomic force microscope to monitor Warhol's acrylic portrait of Brooke Hayward as it was cleaned, to make sure dirt and not paint was being removed.
Sustainable Art
Ormsby and others are also working with scientists at Dow Chemical to use the company's industrial-scale abilities to run a large number of chemical reactions quickly to test a variety of microemulsions on acrylic paint samples. Their goal is to try different combinations of cleaning compounds to find the best formula for washing painting surfaces without harming them.
Plastics researchers are also reaching out to artists to let them know about the potential pitfalls of producing art from plastic. “The idea is not to interfere with the creative process but to allow the artists the option to use this information if they wish to,” says Carolien Coon, who is an artist herself, as well as a conservation scientist at the U.C.L. Institute for Sustainable Heritage. Coon says she wonders about a sculpture she sold years ago that was made of silicone rubber, a bronze cast, a fishbowl and baby oil. “I have no idea how it looks today. I hope it hasn't leaked all over the dining-room table.”
The great hope of conservation scientists is that restoring the past will also help them prepare for the future, when today's plastic materials—such as 3-D-printed objects—start entering museum collections. One such item might be the first 3-D-printed acoustic guitar or a retired International Space Station suit. Eventually all will be past their prime, and conservators want to have the tools in hand to give these cultural icons a face-lift.